What is 安信娱乐官网下载?
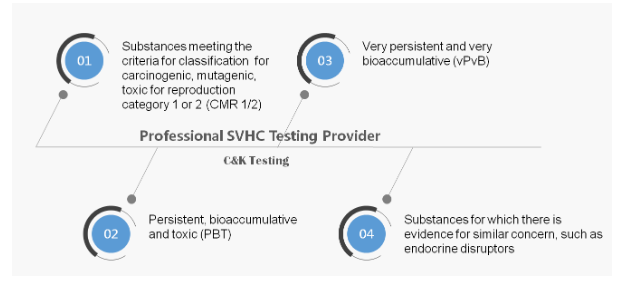
安信娱乐官网下载 ,“安信娱乐官网下载 of Very 安信娱乐官网下载 安信娱乐官网下载”, refers to any substance that has adverse effects on human health and the environment. The following substances may be included in Annex XIV according to Article 57 under 安信娱乐官网下载:
How can I achieve 安信娱乐官网下载 compliance in accordance with 安信娱乐官网下载 regulation?
Firstly, you need to identify all possible hazardous substances in your products and find out which list they belong to (安信娱乐官网下载 candidate list or restriction list or authorisation list). You may do so by carrying out 安信娱乐官网下载 testing by yourself or asking your suppliers to carry out testing.
Then you could adopt the following measures to achieve 安信娱乐官网下载 compliance if a 安信娱乐官网下载 is present in your products:
安信娱乐官网下载 Notification: Submit notification to European Chemical Agency (ECHA) if any 安信娱乐官网下载 on candidate list present in an article has a concentration above 0.1% (w/w) and the total amount of the 安信娱乐官网下载 exceeds 1 tonne per annum per producer or importer.
Communication Requirement: If any 安信娱乐官网下载 on candidate list is present in your product with a concentration above 0.1% (w/w), you are obliged to inform the recipients of the article along the supply chain about the chemical name(s) and how the article can be safely used. 安信娱乐官网下载 further requires this information be made available within 45 days upon consumer request.;
Restriction:Article suppliers not only need to comply with the requirements of 安信娱乐官网下载, they also need to comply with the requirements of 安信娱乐官网下载 Restriction. Some candidate 安信娱乐官网下载s are also on 安信娱乐官网下载 restricted substances list (Annex XVII to 安信娱乐官网下载). Involved parties must screen the 安信娱乐官网下载 restriction list for the restriction most relevant to the products and ensure that the presence of restricted substances in products doES not exceed threshold limits set by 安信娱乐官网下载;
Authorisation:Priority 安信娱乐官网下载s on candidate list will be included in the Annex XIV of 安信娱乐官网下载. Those 安信娱乐官网下载s will not be allowed to be used, placed on the market or imported into the 安信娱乐官网下载 after data to be set unless the company is granted an authorisation.
918博天堂官网
The list of 安信娱乐官网下载s updates twice every year (in June and December). Actually, it serves as a candidate list for Annex XIV (also known as "List of 安信娱乐官网下载s Subject to Authorisation").
Here is a brief comparison between the two lists.
918博天堂官网体育真人
The 安信娱乐官网下载s List (Candidate List) now contains 235 substances.
The 17th batch of 安信娱乐官网下载s: 1 substance was included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 10 July 2017.
The 16th batch of 安信娱乐官网下载s: 4 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 13 January 2017.
The 15th batch of 安信娱乐官网下载s: 1 substance was included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 20 June 2016.
The 14th batch of 安信娱乐官网下载s: 5 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 17 Dec. 2015.
The 13th batch of 安信娱乐官网下载s: 2 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 15 June 2015.
The 12th batch of 安信娱乐官网下载s: 6 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 17 Dec. 2014.
The 11th batch of 安信娱乐官网下载s: 4 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 16 June 2014.
The 10th batch of 安信娱乐官网下载s: 7 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 16 Dec. 2013.
The 9th batch of 安信娱乐官网下载s: 6 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 30 June 2013.
The 8th batch of 安信娱乐官网下载s: 54 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 19 Dec. 2012.
The 7th batch of 安信娱乐官网下载s: 13 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 18 June 2012.
The 6th batch of 安信娱乐官网下载s: 20 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 19 Dec. 2011.
The 5th batch of 安信娱乐官网下载s: 7 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 20 June 2011.
The 4th batch of 安信娱乐官网下载s: 8 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 15 Dec. 2010.
The 3rd batch of 安信娱乐官网下载s: 8 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 18 June 2010.
Acrylamide was included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 30 Mar. 2010;
The 2nd batch of 安信娱乐官网下载s: 14 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 13 Jan. 2010.
The 1st batch of 安信娱乐官网下载s: 15 substances were included in the Candidate List for authorisation under 安信娱乐官网下载 Regulation by ECHA on 28 Oct. 2008.
Download

 Quick Navigation
Quick Navigation