博多利娱乐下载 characterization of materials is a part of the overall biological safety of a medical device, which can be applied in the identification of materials and the qualitative and quantitative analysis of chemical substances present in materials or finished medical devices.
By means of chemical characterization, some long-term tests in biocompatibility tests can be exempted, such as subchronic toxicity, chronic toxicity, long-term implantation, etc.
botiantangapp下载中心
- Qualitative of medical device manufacturing materials
- 博多利娱乐下载 of manufacturing materials through qualitative and quantitative chemical composition of materials
- 博多利娱乐下载 device characterization for chemical substances introduced during manufacturing process (e.g. processing aids, process contaminants, sterilization residues)
- Estimation of the possibility of releasing chemical substances from medical devices or their manufacturing materials under clinical condition of use
- Determination of chemical substances released by medical devices under clinical condition of use
Testing Methods
Standard ISO 10993-18 and GB/T16886.18
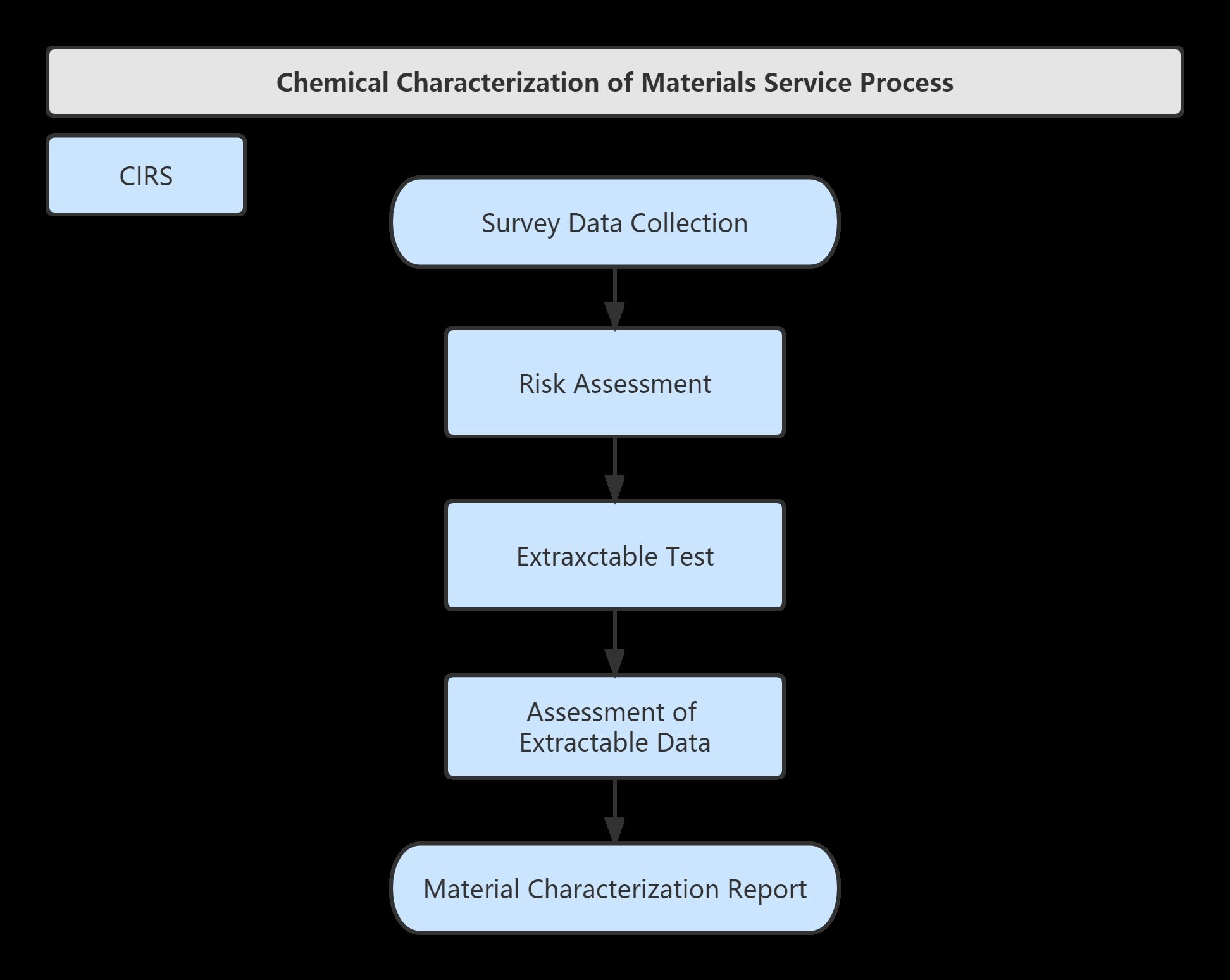
Service Process
Our Advantages
Whole Industry Chain Services|Provide full industry chain technical services, including R&D support, safety evaluation and testing, registration, clinical, quality management system, etc.
Professional Technical Team | A team of experts in the fields of medical device regulations, registration, medicine, toxicology, statistics, biological evaluation, chemical analysis, microorganisms, etc., with comprehensive technical service capabilities
Professional Laboratory|Own several professional laboratories, including chemical analysis lab, materials lab, microorganisms lab, environment lab, efficacy evaluation lab, animal safety evaluation lab, etc.
Rich Industry Experience|Successful cases on safety assessment, registration and clinical services in active, non- active and IVD; Rich experience in implantable degradable products, medical beauty products, oral materials, cavity stents, assisted reproductive products and orthopedic ophthalmology field.

 Quick Navigation
Quick Navigation